30+
Medical Air Systems
Reliable, Hygienic, and Continuous Medical Air Supply
OXYVITAL Medical Air Systems provide continuous, safe, and high-purity air supply for sensitive healthcare environments such as hospitals, operating rooms, surgical intervention areas, intensive care units, dental clinics, and outpatient clinics. Our systems are fully compliant with international standards such as EN ISO 7396-1, HTM 02-01, and the European Pharmacopoeia.
What makes air “medical”?
Medical Air Units are classified as medical devices and therefore must be designed and manufactured in compliance with ISO 13485 – Quality Management Standard for Medical Devices. Since medical air supply is critical for patient safety, air generation systems must comply with the following regulations and international standards:
Medical Device Regulation (MDR) EU 2017/745
EN ISO 7396-1, HTM 02-01 or HTM 2022 (Europe)
Medical air purity criteria
* O₂: 19.5–23.5 %
* CO₂ ≤ 500 ppm
* CO ≤ 5 ppm
* Oil vapor ≤ 0,1 mg/m³
* Particle and bacteria limits
* CO₂ ≤ 500 ppm
* CO ≤ 5 ppm
* Oil vapor ≤ 0,1 mg/m³
* Particle and bacteria limits
Medical Air Systems
Key Features
Medical Air Systems
Medical Air Units are classified as medical devices and therefore must be designed and manufactured in compliance with ISO 13485 – Quality Management Standard for Medical Devices.
Since medical air supply is critical for patient safety, air generation systems must comply with the following regulations and international standards:
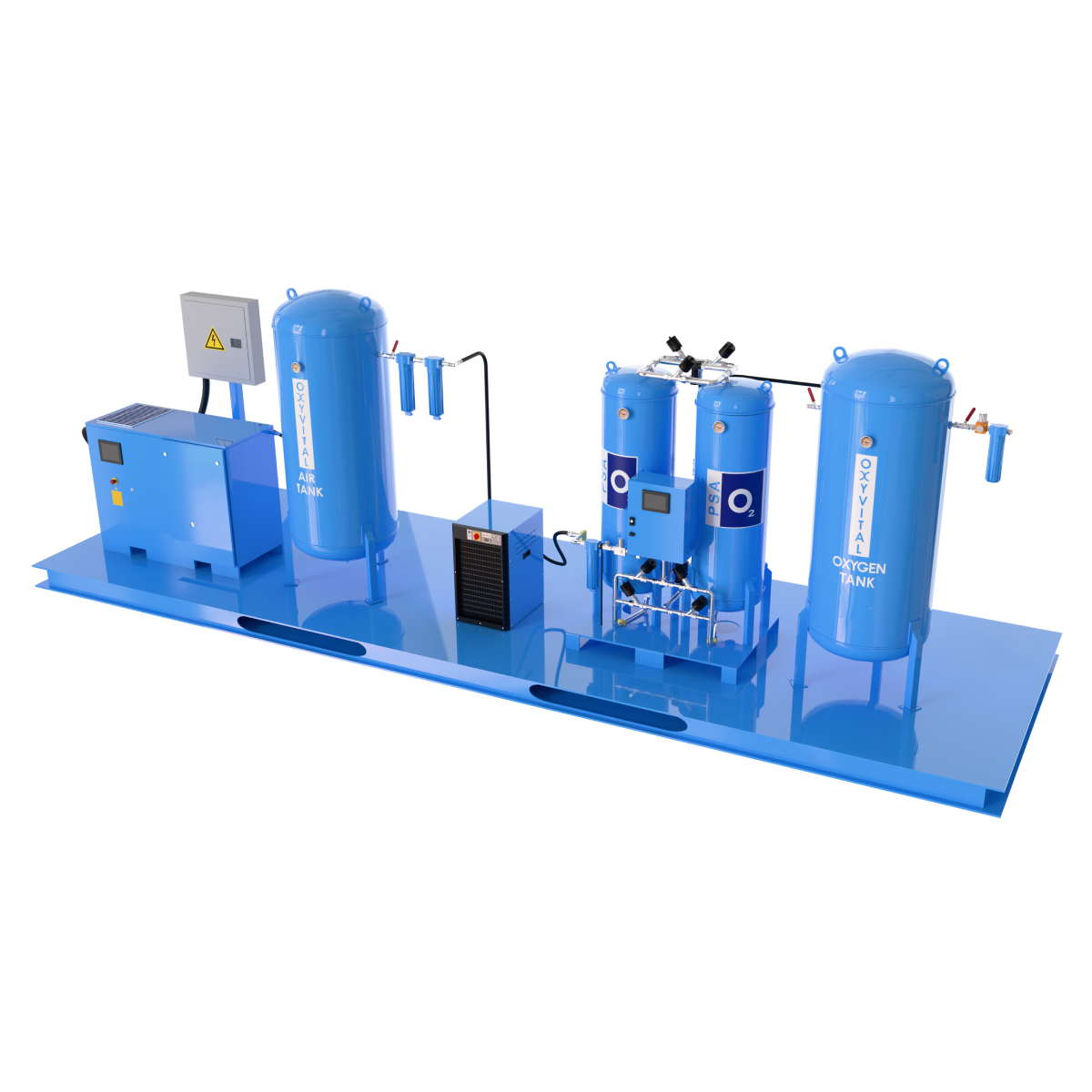
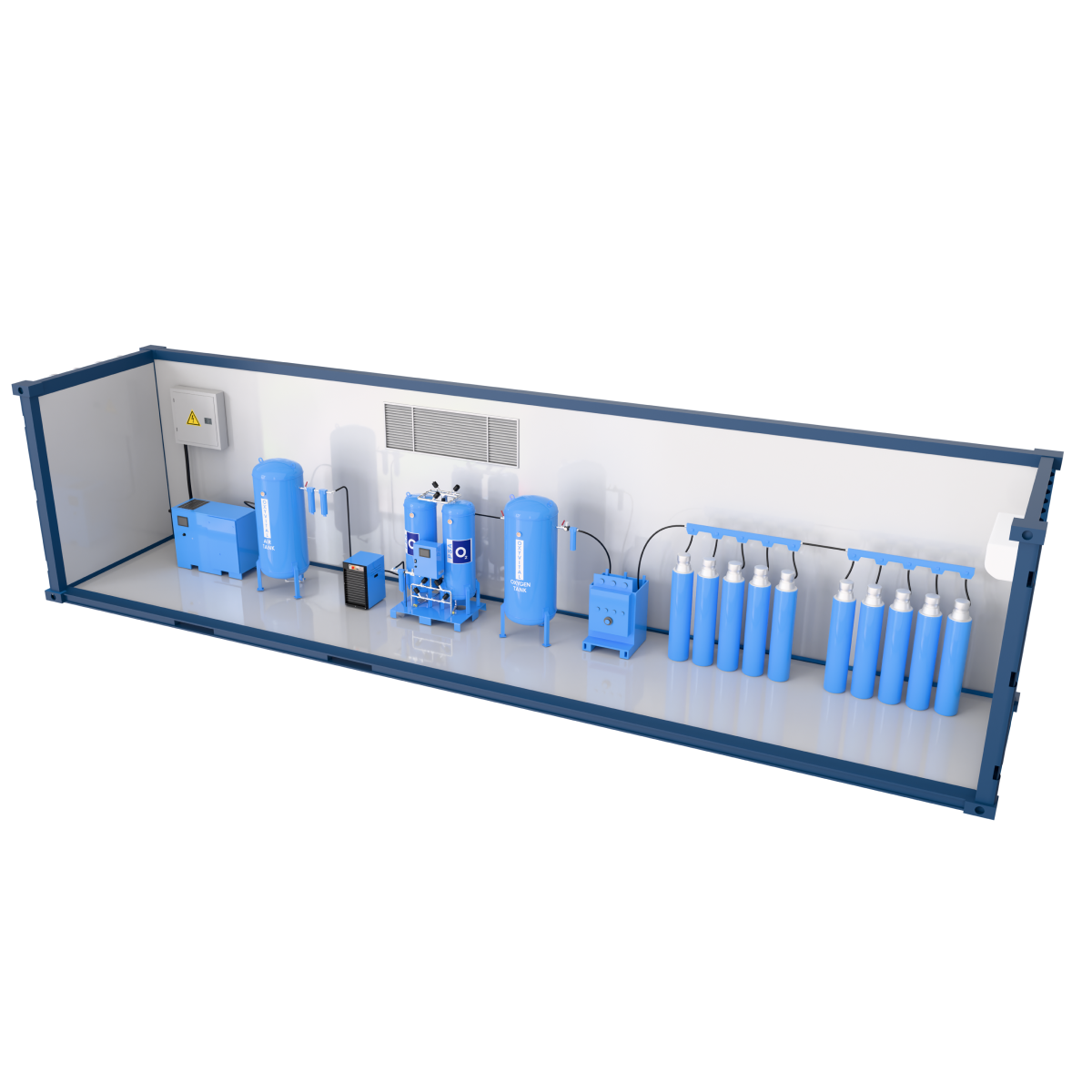
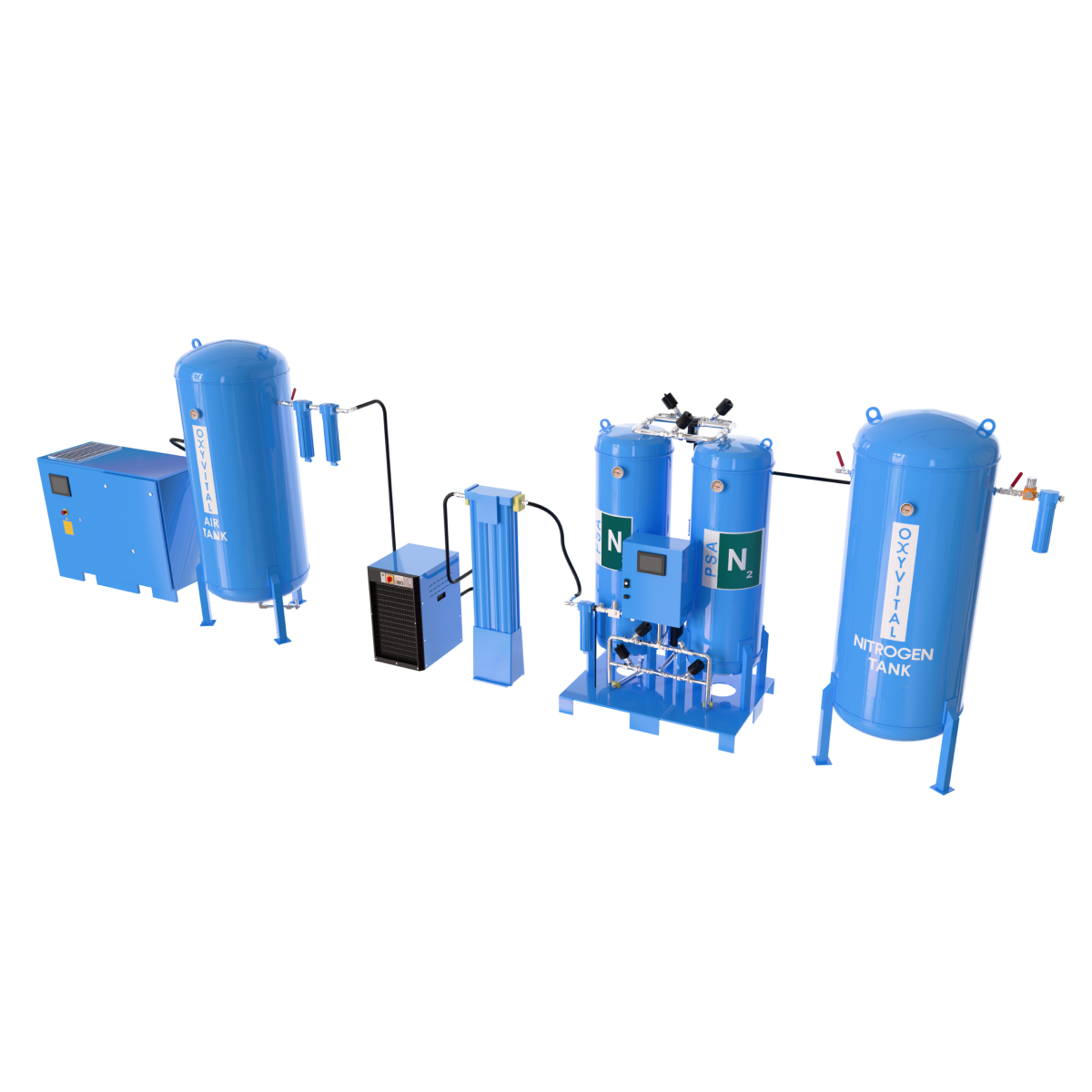
System Components
Air Compressor Group
Typically 2 or 3 units in N+1 configuration
Air Dryer
Refrigerant or Adsorption Type
Filters
Dust, activated carbon, bacterial
Air Backup Tank
Activated Carbon Tower
The oil vapor from the compressor (which may be present in small amounts even if oil-free), as well as traces of exhaust gases in the ambient air (CO, NOx, etc.), are eliminated to prevent odor and taste disturbances.
This is essential to meet the ≤ 0.1 mg/m³ oil vapor limit specified in the Pharmacopoeia.
Control Panel
Automatic Operation and Backup System Activation
STERILE / Bacterial Filter
At the final outlet line